
(1970), The Mathematical Theory of Non-Uniform Gases (3rd ed.), Cambridge University Press In the following table, the temperature is given in kelvins. Viscosity of water as a function of temperature Substance Substances of variable composition Substance

This is also the reason oils tend to be highly viscous, since they are usually composed of long-chain hydrocarbons.
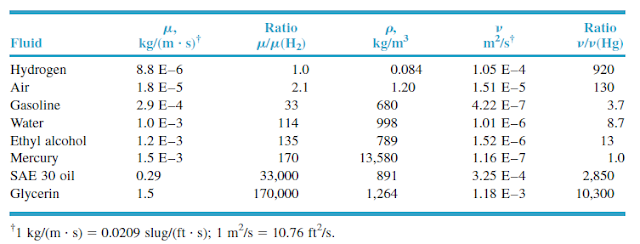
More dramatically, a long-chain hydrocarbon like squalene (C 30H 62) has a viscosity an order of magnitude larger than the shorter n-alkanes (roughly 31 mPa This effect can be observed for the n-alkanes and 1-chloroalkanes tabulated below. Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow. One of the key predictions of the theory is the following relationship between viscosity μ For this reason, measured viscosities of the noble gases serve as important tests of the kinetic-molecular theory of transport processes in gases (see Chapman–Enskog theory). The simple structure of noble gas molecules makes them amenable to accurate theoretical treatment. By contrast, pressure is omitted since gaseous viscosity depends only weakly on it. The temperatures corresponding to each data point are stated explicitly. Where data points are unavailable for 25 ☌ or 1 atmosphere, values are given at a nearby temperature/pressure. Here "standard conditions" refers to temperatures of 25 ☌ and pressures of 1 atmosphere. Viscosities at or near standard conditions 2 Viscosities under nonstandard conditions.1 Viscosities at or near standard conditions.A scaling analysis based on Navier-Stokes equations is presented at the end, and the predicted θ c matches with experimental observations without any additional fitting parameters. Furthermore, we observed a viscous wetting regime only on surfaces with an equilibrium contact angle θ eq smaller than a critical angle θ c depending on viscosity. For relatively high viscosity liquids, the inertial wetting time increases with liquid viscosity, which may due to the viscous damping of the surface capillary waves. For low viscosity liquids, the duration of inertial wetting corresponds to the time of capillary wave propagation, which can be determined by Lamb's drop oscillation model for inviscid liquids. It was further found that surface wettability does not affect the duration of inertial wetting, whereas the viscosity of the liquid does.
.png)
In contrast, the exponent of the power law does only depend on surface wettability as also reported in literature. In the early inertial wetting regime, the coefficient of the wetting power law increases with surface wettability but decreases with liquid viscosity. We show that surface wettability and liquid viscosity influence wetting dynamics and affect either the coefficient or the exponent of the power law that describes the growth of the wetting radius.

Solid surfaces with different lyophilic and lyophobic coatings (equilibrium contact angle θ eq of 0°–112°) were used to study the effect of surface wettability. Drop of glycerol water mixtures and pure water that have comparable surface tensions (62.3–72.8 mN/m) but different viscosities (1.0–60.1 cP) were used. In this paper, we experimentally investigated the dynamic spreading of liquid drops on solid surfaces.


 0 kommentar(er)
0 kommentar(er)
